Embarking on a scientific expedition to unravel the secrets of molar mass can be an exhilarating adventure. Whether you’re a seasoned chemist or a budding enthusiast, this comprehensive guide will navigate you through the intricacies of this fundamental concept, arming you with the knowledge to conquer any molar mass challenge that comes your way.
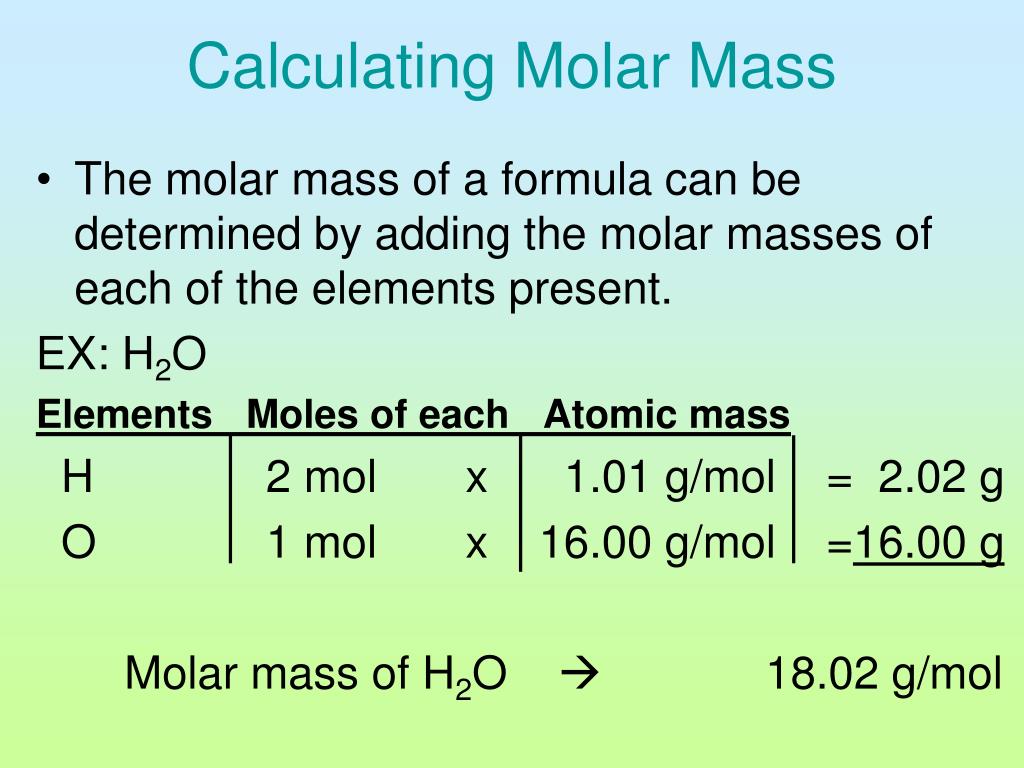
Image: haipernews.com
Unlocking the Mystery: What is Molar Mass?
At the heart of our quest lies molar mass, an essential tool for understanding the composition and behavior of substances. It represents the mass of one mole of a substance, a mole being a colossal unit equal to 6.022 × 1023 entities, which could be atoms, molecules, ions, or any other fundamental building blocks.
Knowing the molar mass unlocks a treasure trove of information about a substance. It allows us to determine its elemental composition, predict its reactivity, and calculate crucial quantities such as concentration and purity.
Charting the Path: A Step-by-Step Guide to Calculating Molar Mass
Mastering the art of molar mass calculation requires a systematic approach, meticulously navigating the following steps:
1. Identify the Elements and Their Abundance:
The first step involves identifying the elements present in the substance and their respective proportions. This information can be gleaned from the chemical formula or molecular structure.
Image: www.myxxgirl.com
2. Retrieve Atomic Masses from the Periodic Table:
For each element, locate its atomic mass, which represents the average mass of all its naturally occurring isotopes. These values can be found in the periodic table.
3. Multiply Atomic Masses by the Number of Molar Masses:
Next, multiply the atomic mass of each element by its corresponding number of moles. In most cases, this is determined directly from the chemical formula.
4. Summing Up the Masses:
Finally, add up the products obtained in step 3. The resulting sum represents the molar mass, expressed in grams per mole.
Harnessing the Power of Molar Mass: Applications in Various Fields
The practical applications of molar mass span a diverse range of fields, including:
1. Chemistry:
In chemistry, molar mass plays a pivotal role in stoichiometry, enabling the calculation of quantities such as reactants and products in chemical reactions. It also aids in determining the molecular weight of compounds and understanding their behavior in solution.
2. Material Science:
Molar mass is crucial in materials science for characterizing polymers and other complex materials. It helps determine their physical properties, such as strength, elasticity, and density, guiding the design and synthesis of advanced materials.
3. Biochemistry:
In biochemistry, molar mass is essential for understanding the structure and function of biological molecules. It facilitates the measurement of molecular weights, enzyme kinetics, and the analysis of metabolic pathways.
How To Calculate The Molar Mass Of An Element
Unveiling the Treasure Trove of Knowledge: Conclusion
Mastering the calculation of molar mass opens a gateway to a wealth of scientific knowledge. By understanding this fundamental concept, you gain the power to decipher the composition and behavior of substances, lay the groundwork for advanced research, and harness its practical applications in diverse fields. Embark on this scientific expedition with confidence, and may your journey be filled with discovery and enlightenment.

/GettyImages-1303637-two-way-mirror-57126b585f9b588cc2ed8a7b-5b8ef296c9e77c0050809a9a.jpg?w=740&resize=740,414&ssl=1)



