In the intricate world of chemistry, understanding the concept of valence electrons is crucial. These electrons, perched on the outermost energy level of an atom, dictate its chemical behavior and determine its reactivity. Without a firm grasp of valence electrons, unraveling the mysteries of chemistry can be an elusive endeavor.
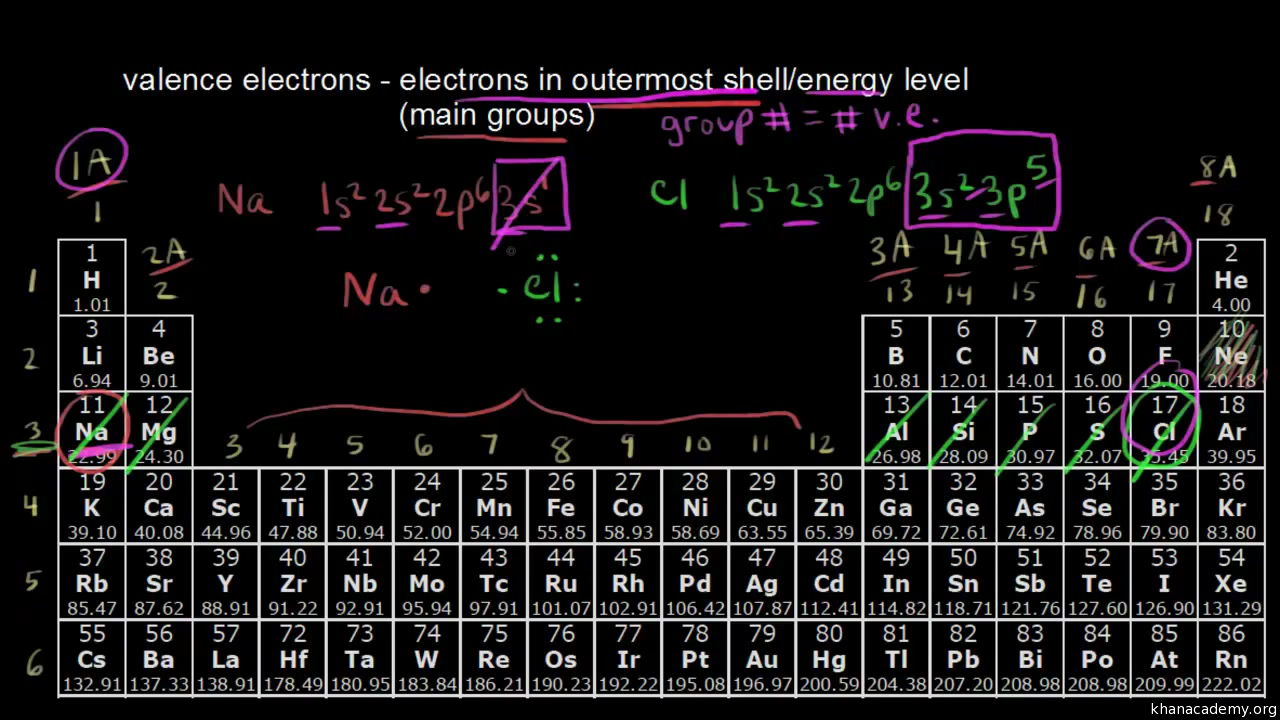
Image: bejaysamael.blogspot.com
Allow this detailed guide to illuminate the path towards uncovering the elusive nature of valence electrons, empowering you with the knowledge to conquer chemical equations and unveil the wonders of the atomic realm.
Delving into the Quantum Realm: What are Valence Electrons?
Envision an atom as a miniature solar system, with the positively charged nucleus as the central star and negatively charged electrons orbiting it at varying distances. These electrons occupy specific energy levels or shells, and those residing in the outermost shell are known as valence electrons.
Valence electrons hold the key to an atom’s chemical identity. They are the players in the intricate dance of bonding, the force that holds atoms together to form molecules. Their number and arrangement bestimmen the atom’s reactivity, shaping its interactions with neighboring atoms.
Unveiling the Secrets: Methods to Determine Valence Electrons
- Periodic Table Trends: The periodic table, a chemist’s road map, provides a wealth of information about an element’s valence electrons.
- Elements within the same group share the same number of valence electrons. For instance, all alkali metals (Group 1) possess one valence electron, while halogens (Group 17) boast seven.
- Electron Dot Diagrams: These pictorial representations depict valence electrons as dots surrounding the element’s symbol. For example, sodium, with one valence electron, is represented as:
Na.- Orbital Configuration: This more advanced method involves examining the atom’s electron configuration, a detailed description of the orbitals occupied by electrons. The number of valence electrons equals the electrons in the outermost occupied orbital.
Harnessing the Power of Valence Electrons: Applications Galore
Mastering the art of determining valence electrons unlocks a treasure trove of applications.
-
Predict Chemical Reactivity: By knowing an element’s valence electrons, one can anticipate its ability to form bonds. Elements with a high number of valence electrons are more reactive, readily participating in chemical reactions.
-
Unveiling Compound Formation: Valence electrons play a pivotal role in determining how atoms combine to form compounds. Understanding their number guides the understanding of molecular structure and properties.
-
Fueling the Future: Batteries and Solar Cells: Valence electrons hold the key to understanding the principles underlying energy storage in batteries and the conversion of sunlight into electricity in solar cells.

Image: sciencenotes.org
How To Find Amount Of Valence Electrons
Conclusion: The Cornerstone of Chemistry
Valence electrons, the gatekeepers of chemical reactivity, form the cornerstone of chemistry. Determining their number and arrangement provides a profound understanding of an element’s behavior and empowers us to predict its interactions with others.
By embracing the techniques outlined above, you possess the tools to navigate the complexities of chemistry with confidence. Remember, the journey of a thousand chemical equations begins with a single valence electron. Master this concept, and the world of chemistry will unfold before you like an unraveling tapestry of discovery.


/GettyImages-1303637-two-way-mirror-57126b585f9b588cc2ed8a7b-5b8ef296c9e77c0050809a9a.jpg?w=740&resize=740,414&ssl=1)


